Science with Passion
Comparison of organic compounds in natural wine, red wine and grape juice by HPLC
J. Wesolowski, Y. Krauke, J. Kramer, G. Greco; krauke@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin

Foto: pixabay; WolfBlur
Summary
Natural wine is the latest trend in the field of wine lovers. It is a wine produced by natural fermentation of grape juice without the use of external additives and it is becoming a popular beverage among the young generations. But what is the difference from classical wine? Here, we investigated the sugars, alcohols, organic acids and phenolic compounds in natural wine, red wine and grape juice with two simple and rapid chromatographic methods. For the determination of the most important organic acids, sugars and alcohols in wine and grape juice, a method was developed using an ion exchange column, a refractive index detector (RID) and a diode array detector (DAD). Furthermore, a reversed-phase LC method was developed and optimized for the determination of phenolic compounds using the DAD. The combination of two different separation modes with two different detectors in one system enabled the qualitative detection of approx. 20 substances in wine and juice.
Introduction
Analytical methods are essential to ensure product quality, execute regulations and conform with food standards, specifications and labeling requirements in the food industry. High performance liquid chromatography (HPLC) is one of the most important and widely used methods in this field.
With a wide range of column materials and detectors, it can be used to analyse various compounds. The detection of sugars, alcohols, organic acids, phenolic acids and flavonoids is part of the daily routine in beverage analysis and represents an important application. An overview of exemplary analytes in beverage analysis is shown in Fig. 1.
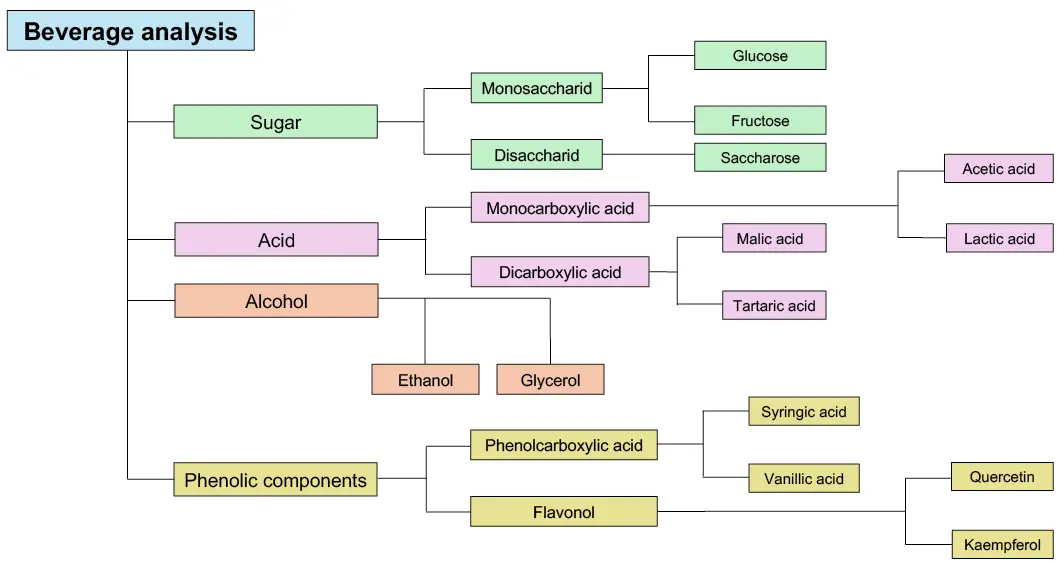
Fig. 1 Overview of common analytes in the beverage analysis.
White wine, red wine or natural wine (also known as unadulterated wine, naked wine, raw wine, minimal or low intervention wine) is defined as an alcoholic beverage produced by the fermentation of fruit [1]. Grapes are the most cultivated fruits for the production of wine [1]. Sugar, organic acids, alcohols and phenolic compounds are the main components of this fruit [1]. The most important sugars found in grapes are glucose and fructose. During alcoholic fermentation by yeasts, the glucose is fermented first and then fructose. When the wine grapes are completely fermented, the wine contains nearly no fructose or glucose [2]. The alcohol in wine is produced during alcoholic fermentation, which is caused by enzymes. Wine mainly contains ethanol, but glycerin, which is found in very ripe grapes, is also important2. Organic acids, such as malic acid and tartaric acid, which are mainly found in wine, are already present in the grapes, while others, such as acetic acid and lactic acid, are only generated during the fermentation process [2]. The most important phenolic compounds in wine include the groups of flavonoids and phenolic acids. These contain, for example quercetin and vanillin acid. The levels of all these components in wine not only affect the taste balance and optical appearance, but also the chemical stability, pH value, and overall quality of the wine [3]. Therefore, it is crucial for quality assurance and process control to measure these different substances present in wine [3].
Sample Preparation
Sugar/ Alcohol/ Organic acid
All standards (sucrose, glucose, fructose, glycerol, ethanol, sorbitol, tartaric acid, lactic acid, acetic acid, citric acid, succinic acid, malic acid), except for shikimic acid and the standard mixes, were dissolved in distilled water to a concentration of 2 mg/ml. The shikimic acid was dissolved in distilled water to a concentration of 0.8 mg/ml.
Phenolic acid/ Flavonoid
All standards (syringic acid, vanillic acid, p-coumaric acid, ferulic acid, benzoic acid, quercetin), except for kaempferol were dissolved in water/acetonitrile 50:50 (v/v) to a concentration of 1 mg/ml. Kaempferol was dissolved in in water/acetonitrile 50:50 (v/v) to a concentration of 0.5 mg/ml. Before injection, the samples were diluted to a concentration of 0.1 mg/ml. The standard mix was made from the individual standards (1 mg/ml) and dissolved in water/acetonitrile 50:50 (v/v) to a concentration of 0.1 mg/ml.
Natural wine/ Red wine/ Grape juice
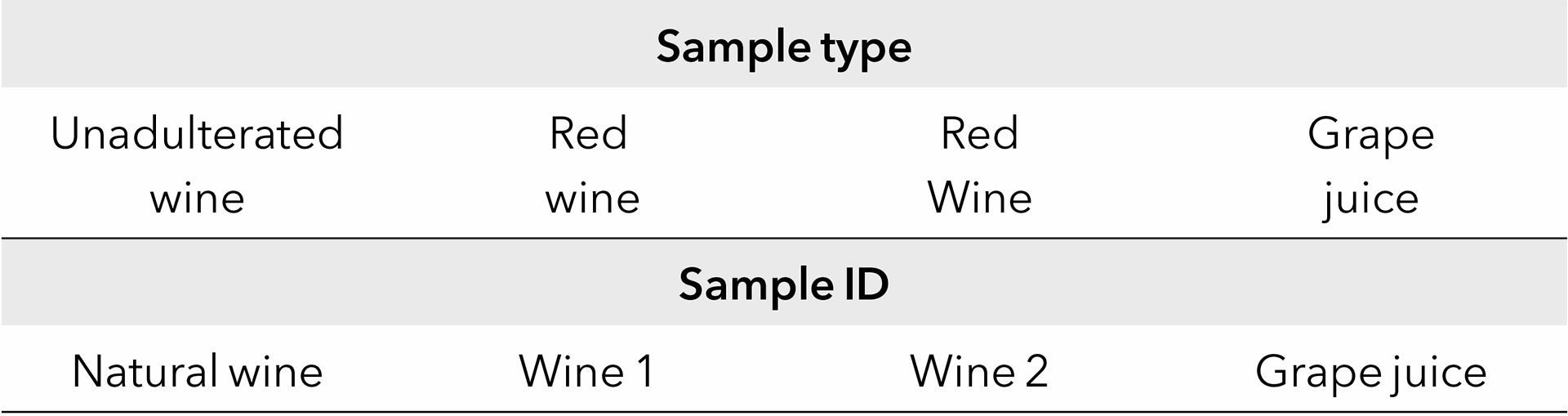
Before injection, the samples (Tab. 1) were filtered with a CA (cellulose acetate) filter (0.45 µm).
Tab. 1 Sample description.




Results
Eurokat H
Sugars and organic acids are highly polar. Non-polar reversed-phase columns (RP columns), such as C18 columns, which are used in most HPLC methods, cannot be used due to the lack of interaction with these polar substances. Instead, polymer-based ion exchange/ligand exchange columns (for example KNAUER Eurokat columns) which use water as eluent are applied. This is not only environmentally friendly, but also has the advantage that water soluble sugars can be easily injected.
The Eurokat H column was specially developed for the analysis of organic acids, monosaccharides, alcohols and sugar alcohols. Even complex mixtures of these substances can be separated with this column. Therefore, the development was first carried out with an Eurokat H column. The eluent water was acidified with sulfuric acid to 0.005 M. Various methods were tested to achieve the best resolution between the substances. Different temperatures (40 °C and 60 °C) and flow rates (0.5 ml/min and 0.7 ml/min) were tested and the best separation was achieved with 60 °C and 0.7 ml/min. UV detection was at 210 nm. In order to be able to clearly identify the substances in the sample, all standards were measured separately. The resulting chromatogram with the standards measured with the DAD is shown in Fig. 2.
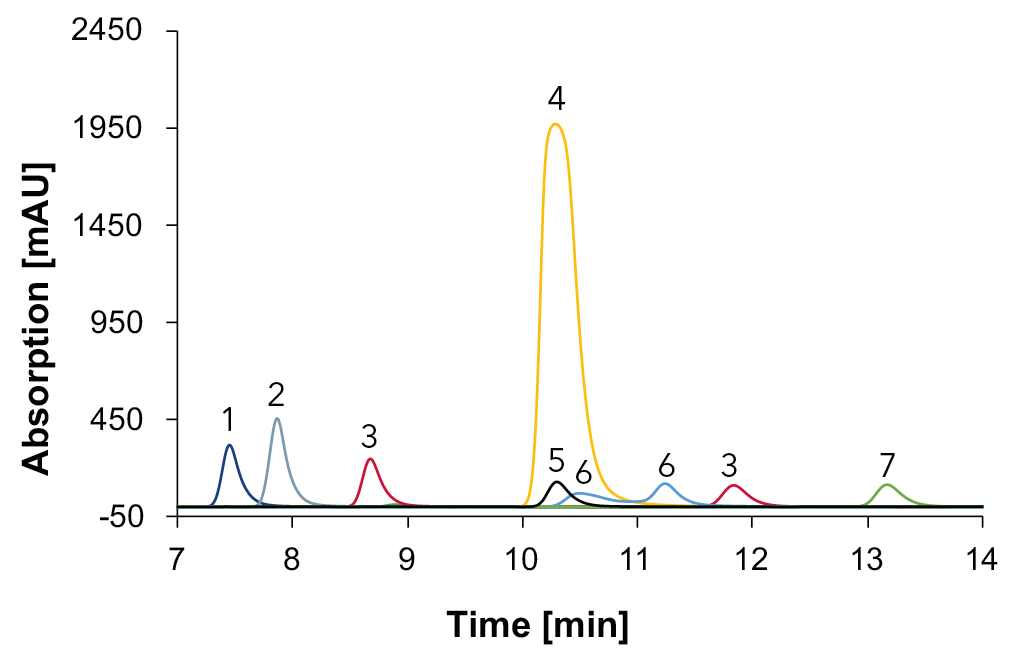
Fig. 2 Overlay chromatograms with standards measured with Eurokat H and DAD at 210 nm, 60 °C, 0.7 ml/min, 1: citric acid, 2: tartaric acid, 3: malic acid, 4: shikimic acid, 5: succinic acid, 6: lactic acid, 7: acetic acid.
It can be seen that all organic acids absorb at 210 nm and can be detected using the DAD. Furthermore, for malic acid (3) two peaks can be seen in the chromatogram: one at 8.68 min and one at 11.84 min. A possible reason for the two peaks is that malic acid usually contains an impurity of fumaric acid, since malic acid is synthesized from fumaric acid4. At 210 nm, fumaric acid absorbs UV light much more strongly than malic acid4.
Therefore, even a small impurity in fumaric acid can result in a peak comparable in size to the malic acid peak4. On this basis, the peak that elutes later is assumed to be fumaric acid4. The appearance of a double peak for lactic acid can be explained by the fact that it exists in two forms (molecular form and ionized form)5.
The 3D absorption spectrum can be used to view the substance-specific absorption spectra at certain wavelengths and helps to differentiate between substances that elute at the same time. For example, the shikimic acid (4) and succinic acid (5) can be distinguished from each other in this way (Fig. 3).
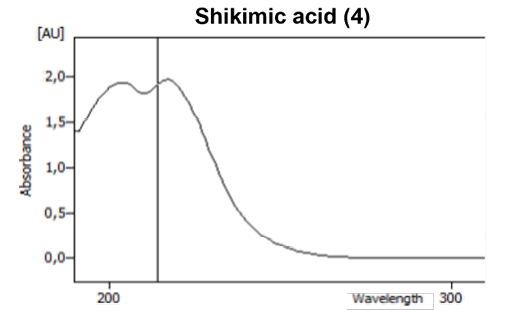
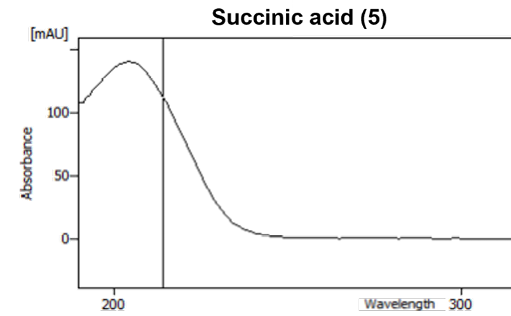
Fig. 3 Absorption spectrum of shikimic acid (4) and succinic acid (5).
Commonly carbohydrates do not have chromophores. Therefore, detection must be based either on the change in the refractive index of the eluate (RID), light scattering of the substances presents in the eluate (ELSD) or the electrochemical properties of carbohydrates. The use of an RID is here the simplest and most cost-effective option. The resulting chromatogram with the standards measured with the RID is shown in Fig. 4.
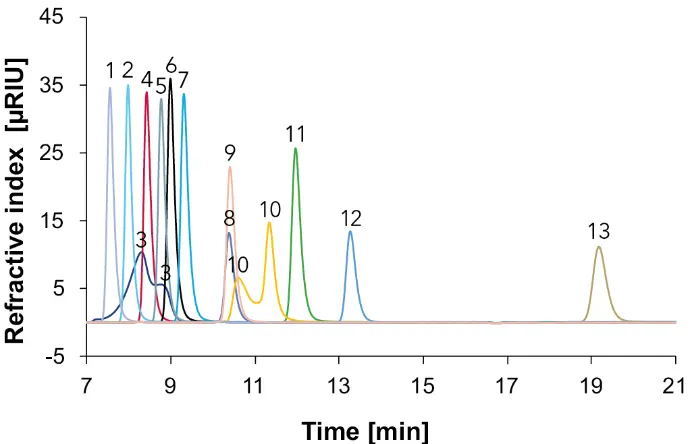
Fig. 4 Overlay chromatograms with standards measured with Eurokat H and RID, 60 °C, 0.7 ml/min, 1: citric acid, 2: tartaric acid, 3: sucrose, 4: glucose, 5: malic acid, 6: fructose, 7: sorbitol, 8: shikimic acid, 9: succinic acid, 10: lactic acid, 11: glycerol, 12: acetic acid, 13: ethanol.
In Fig. 4 can be seen that the disaccharide sucrose (3) is hydrolysed into its monosaccharides. The reaction with water and catalytic acid splits the disaccharide sucrose (3) into glucose and fructose. Furthermore, shikimic acid (8) and succinic acid (9) elute at the same time and lactic acid (10) can be seen as a double peak as in Fig. 2. With the exception of sucrose (3), lactic acid (10) and shikimic acid (8), all remaining substances are well separated from each other. Based on the results, a standard mix of glucose, fructose, glycerol, ethanol, sorbitol, tartaric acid, malic acid, acetic acid, citric acid, succinic acid was prepared for a one-point calibration with 2 mg/ml. Although shikimic acid was not used in the standard mixture, it is differentiated from succinic acid in the sample measurements, using the absorbance spectrum from Fig. 3. The resulting chromatogram with a 2 mg/ml standard mix is shown in Fig. 5. In all following chromatograms two different detection signals were monitored. In blue is the chromatogram detected with the DAD at 210 nm, in red with the RID.
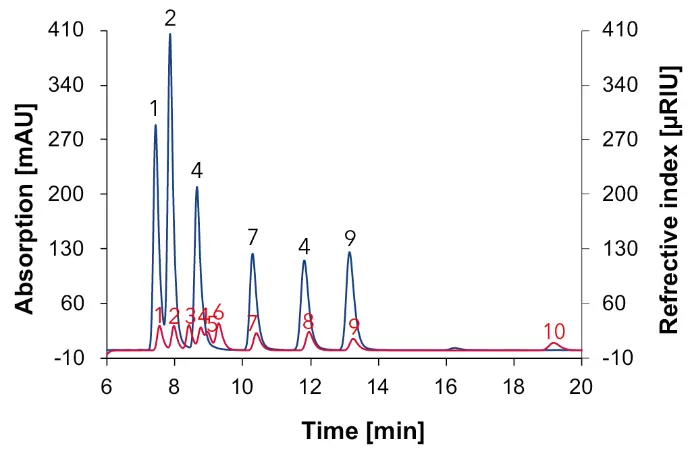
Fig. 5 Chromatogram with a 2 mg/ml standard mix measured with Eurokat H, DAD at 210 nm (blue) and RID (red), 60 °C, 0.7 ml/min, 1: citric acid, 2: tartaric acid, 3: glucose, 4: malic acid, 5: fructose, 6: sorbitol, 7: succinic acid, 8: glycerol, 9: acetic acid, 10: ethanol.
Afterwards the four samples were measured. An overlay between the chromatograms of the sample and the standard mixture of 10 substances allowed the identification of the compounds in the samples. The chromatogram for the natural wine is shown in Fig. 6, for the wine 1 in Fig. 8, for the wine 2 in Fig. 9 and for the grape juice in Fig. 10.
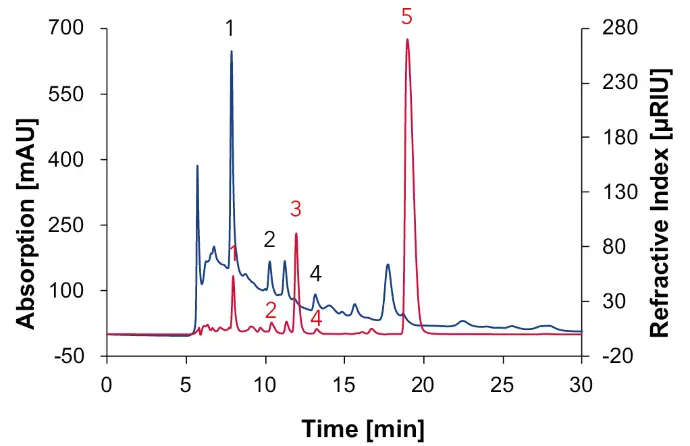
Fig. 6 Chromatogram of natural wine measured with Eurokat H, DAD at 210 nm (blue) and RID (red), 60 °C, 0.7 ml/min, 1: tartaric acid, 2: succinic acid, 3: glycerol, 4: acetic acid, 5: ethanol.
In the natural wine, tartaric acid (1), succinic acid (2), glycerol (3), acetic acid (4) and ethanol (5) can be detected. As expected, no sugar but ethanol is found. To differentiate between succinic acid and shikimic acid, the absorption spectrum from the unidentified peak at 210 nm was used (Fig. 7). The comparison with Fig. 3 shows that shikimic acid has a different absorption spectrum and can therefore be excluded as an acid that is present in natural wine. In addition, the absorption spectrum in Fig. 7 confirms the presence of succinic acid compared to Fig. 3. The differences in absorbance can be explained by matrix effects.
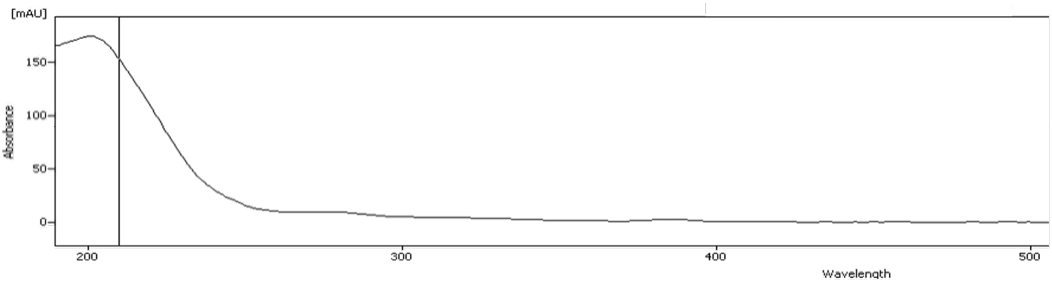
Fig. 7 Absorption spectrum from the unidentified peak at 210 nm.
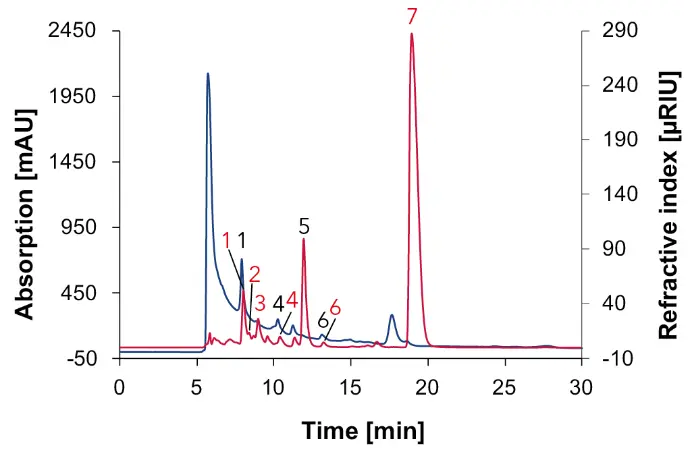
Fig. 8 Chromatogram of wine 1 measured with Eurokat H, DAD at 210 nm (blue) and RID (red), 60 °C, 0.7 ml/min, 1: tartaric acid, 2: glucose, 3: fructose, 4: succinic acid, 5: glycerol, 6: acetic acid, 7: ethanol.
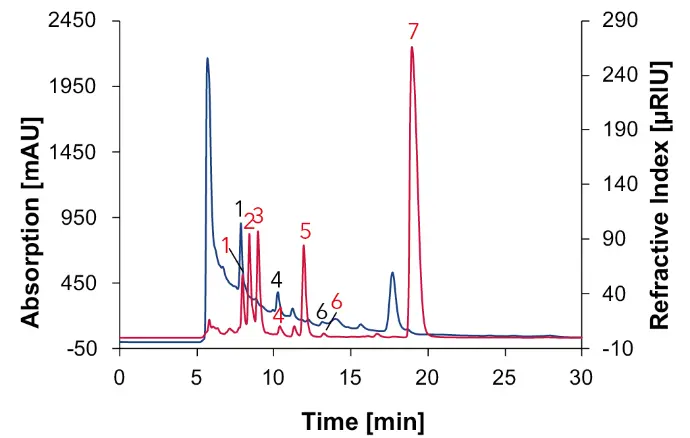
Fig. 9 Chromatogram of wine 2 measured with Eurokat H, DAD at 210 nm (blue) and RID (red), 60 °C, 0.7 ml/min, 1: tartaric acid, 2: glucose, 3: fructose, 4: succinic acid, 5: glycerol, 6: acetic acid, 7: ethanol.
The red wines serve as a reference to the natural wine. As expected in wine 1 and 2, sugars such as glucose (2) and fructose (3) were identified in addition to acids and alcohols, as in natural wine (Fig. 8 and Fig. 9). Similar to natural wine, a high ethanol content was detected in both red wines (Fig. 8 and Fig. 9).
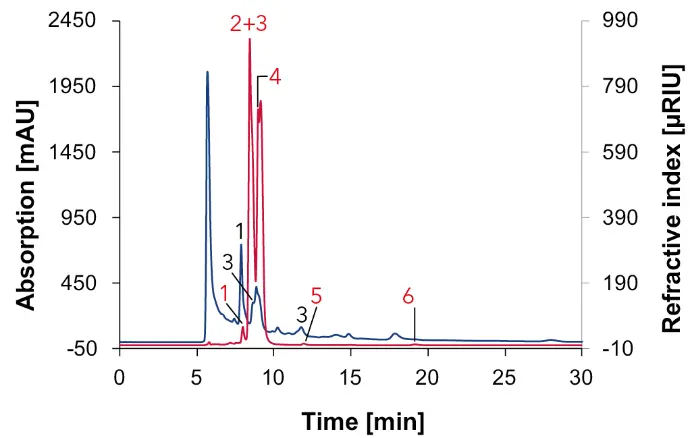
Fig. 10 Chromatogram of grape juice measured with Eurokat H, DAD at 210 nm (blue) and RID (red), 60 °C, 0.7 ml/min, 1: tartaric acid, 2: glucose, 3: malic acid, 4: fructose, 5: glycerol, 6: ethanol.
In addition to sugars like glucose (2) and fructose (4), sugar alcohol such as glycerol (6) was found in the grape juice. The important acids such as tartaric acid (1) and malic acid (3), which are present in grape juice, were identified in the sample. Ethanol (7) is the last substance that can be detected in a small signal, as this can be found not only in alcoholic beverages, but also in low concentrations in different juices.
Eurokat Ca
Furthermore, an Eurokat column in the ionic form Ca was tested to improve sugar separation. The Ca form was developed for the analysis of carbohydrates with DP (degree of polymerization) < 4, and subsequently also for the analysis of mono- and disaccharides. As the eluent water is not acidified separation of organic acids is difficult.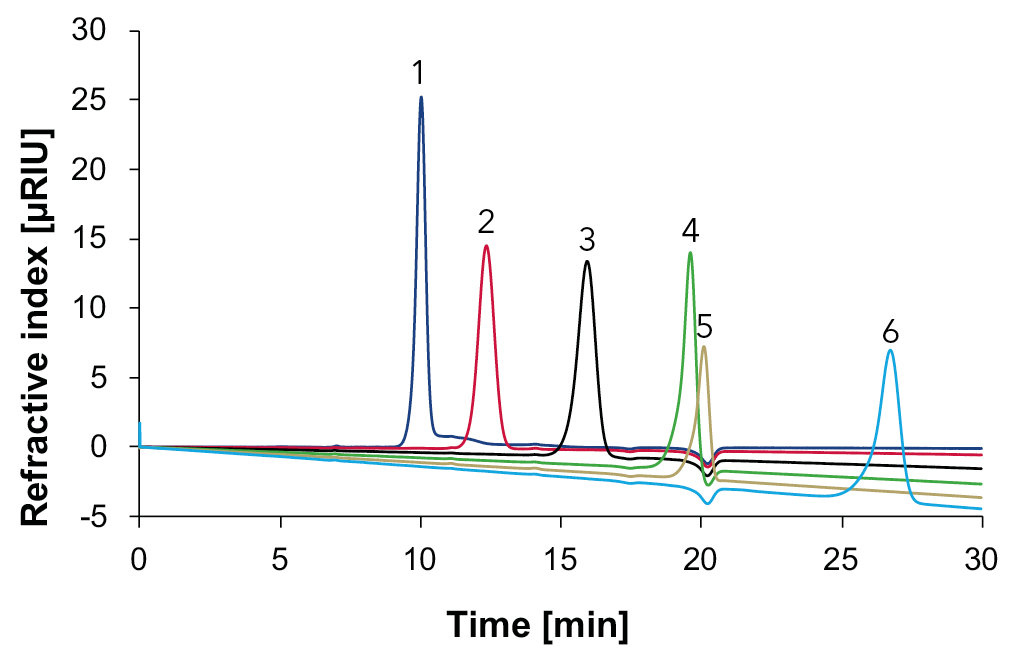
Fig. 11 Overlay chromatograms with standards measured with Eurokat Ca and RID, 75 °C, 0.5 ml/min, 1: sucrose, 2: glucose, 3: fructose, 4: glycerol, 5: ethanol, 6: sorbitol.
As expected, the disaccharide sucrose is not hydrolyzed into its monosaccharides during the measurement with the Eurokat Ca. Furthermore, glycerol (4) and ethanol (5) elute at similar times. If glycerol (4) and ethanol (5) are not considered, all remaining substances are well separated from each other (Fig. 11). In the following, the four samples: wine 1, wine 2, grape juice and natural wine were measured with the Eurokat Ca (Fig. 12).
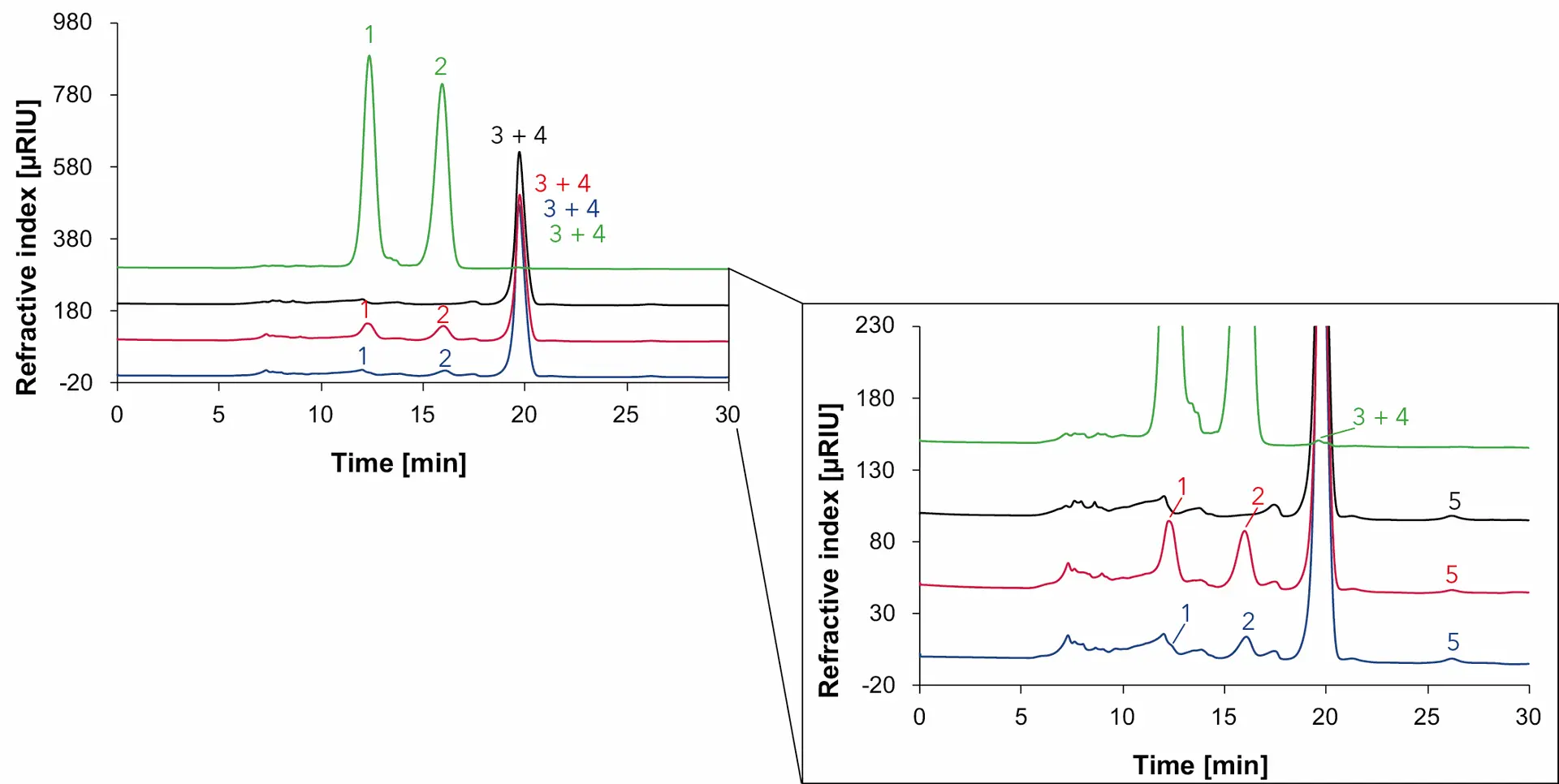
Fig. 12 Overlay chromatograms with samples measured with Eurokat Ca and RID, 75 °C, 0.5 ml/min, blue: wine 1, red: wine 2, black: natural wine, green: grape juice, 1: glucose, 2: fructose, 3: glycerol, 4: ethanol, 5: sorbitol.
In addition to a high content of glucose (1) and fructose (2), in grape juice glycerol (3) and ethanol (4) are also found in very small amounts. In wine 1 and wine 2, on the other hand, glucose (1) and fructose (2) are present in smaller proportions and glycerol (3), ethanol (4) and sorbitol (5) are also detected. Due to the co-elution of glycerol (3) and ethanol (4), the presence of both analytes can be verified on the basis of the sample measurements with the Eurokat H column. In comparison, neither glucose or fructose can be detected in the natural wine. As expected, only glycerol (3), ethanol (4) and sorbitol (5) were found. Due to the co-elution of glycerol (3) and ethanol (4), it can only be presumed that ethanol (4) is found in a much higher quantity than glycerol (3) in natural wine. In general, the results are consistent with the sample measurements optained with the Eurokat H column. The only difference is that sorbitol was not found in the wine measurements with the Eurokat H column compared to those with the Eurokat Ca. It is possible that the signal for sorbitol is too small and it is masked by other substances during the measurement with the Eurokat H.
To get a better understanding of the relative amount of the substances, the signal height of the individual substances in the different samples is compared in Fig. 13. As expected, the fructose and glucose content in the grape juice is significantly higher than in the wine samples while, the ethanol content in the grape juice is significantly lower than in the wine samples. In contrast, the tartaric acid has a similar content in all four samples.
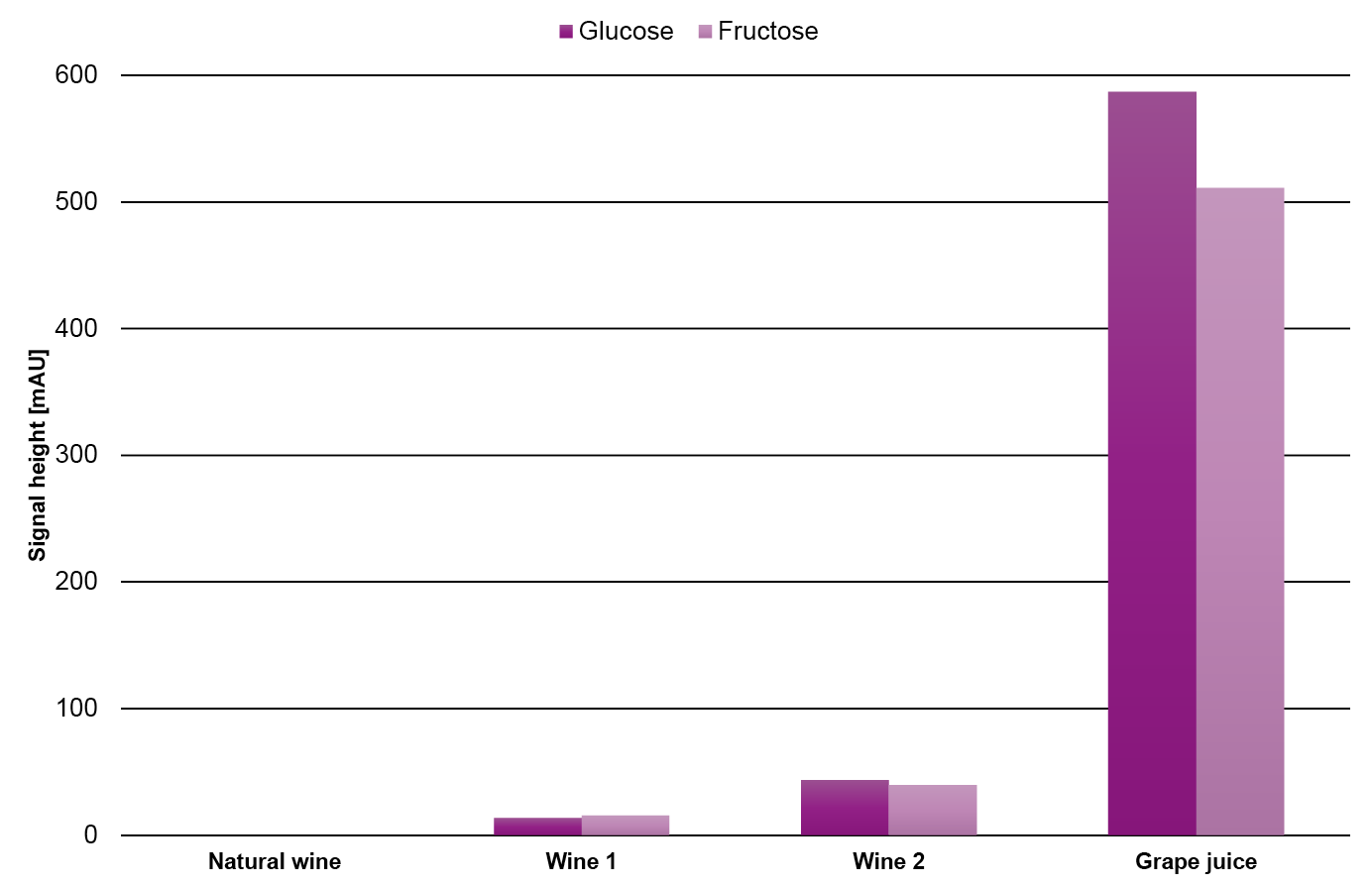
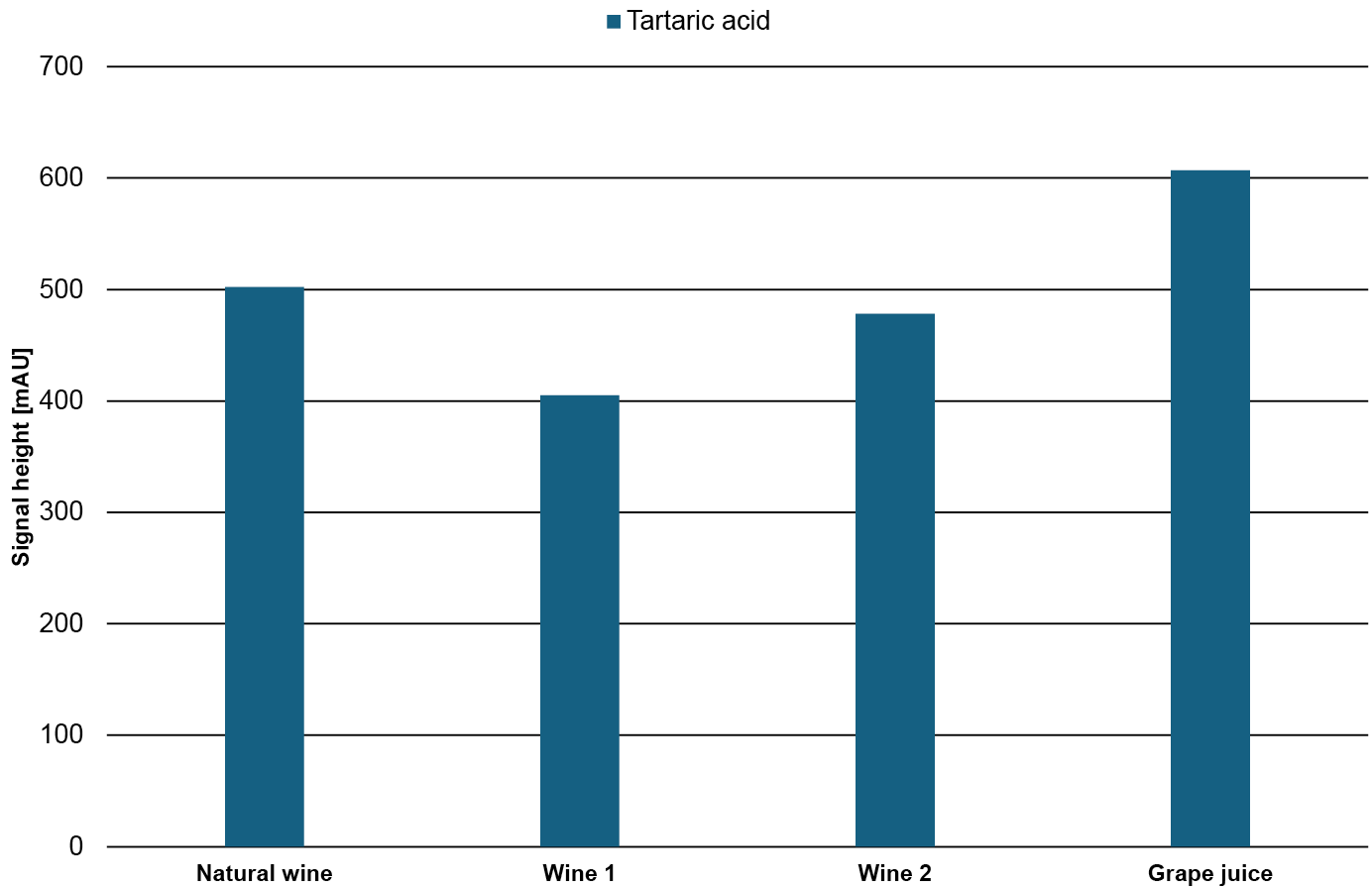
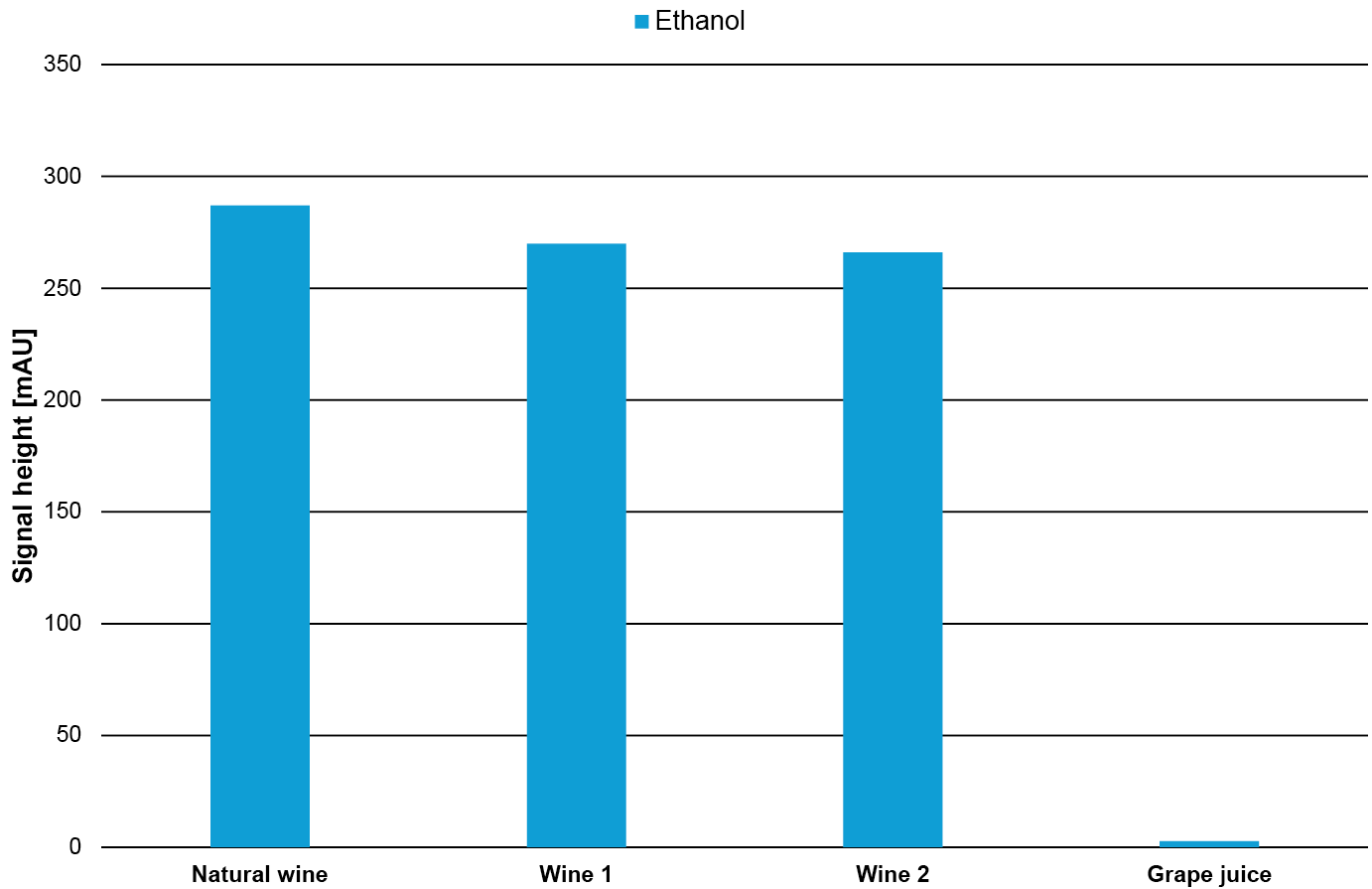
Fig. 13 Signal height of glucose, fructose, tartaric acid and ethanol in the four different samples.
Eurospher II C18
The determination for qualification of phenols/flavonoids was initially carried out with an Eurospher II C18 column. In order to obtain an overview of the retention behavior of the phenols/flavonoids, a linear gradient was applied on the analytical system. Water acidified with 0.2 % phosphoric acid was used as eluent A and acetonitrile was first used for eluent B. The column thermostat was set to 25 °C, a flow rate of 1 ml/min was used and an injection volume of 10 µl was chosen. To clearly identify the substances in the sample, all standards were measured separately. The measurements were carried out with a DAD at the three wavelengths (230 nm/254 nm/275 nm). Subsequently, it was decided to prepare a standard mix of the seven phenols/flavonoids and to measure them. When using acetonitrile as eluent B, the peak resolution of the standard mixture was insufficient. Acetonitrile was replaced by methanol, due to its lower elution strength. The results are shown in Fig. 14.
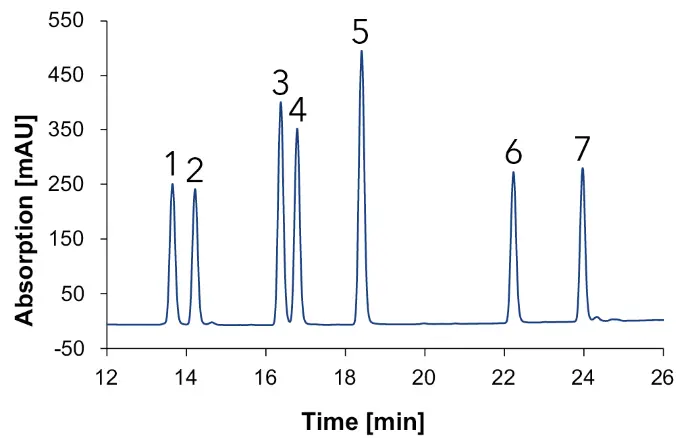
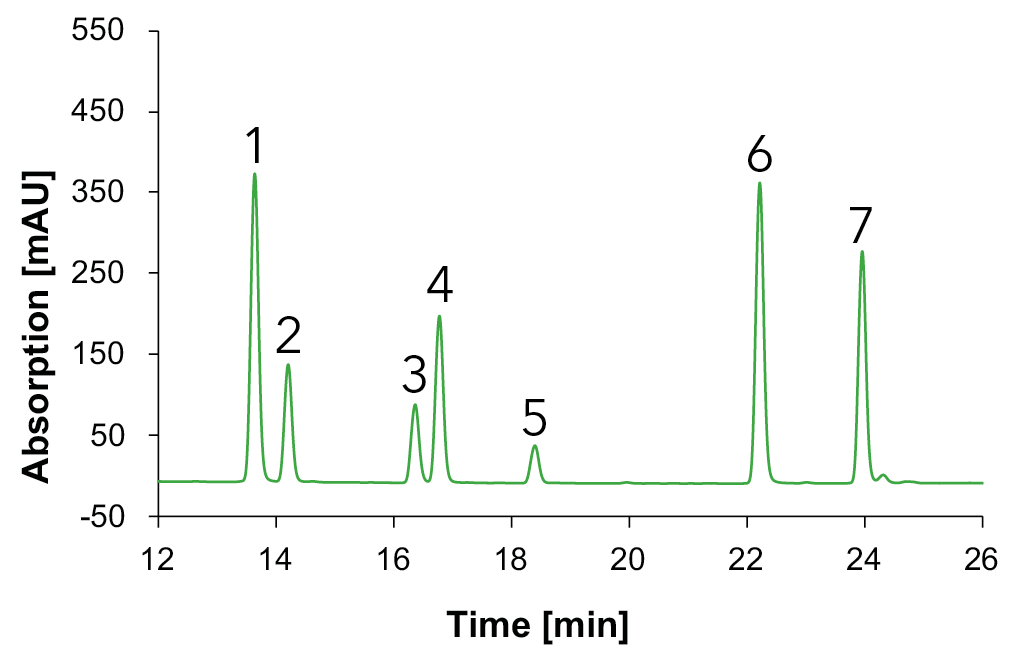
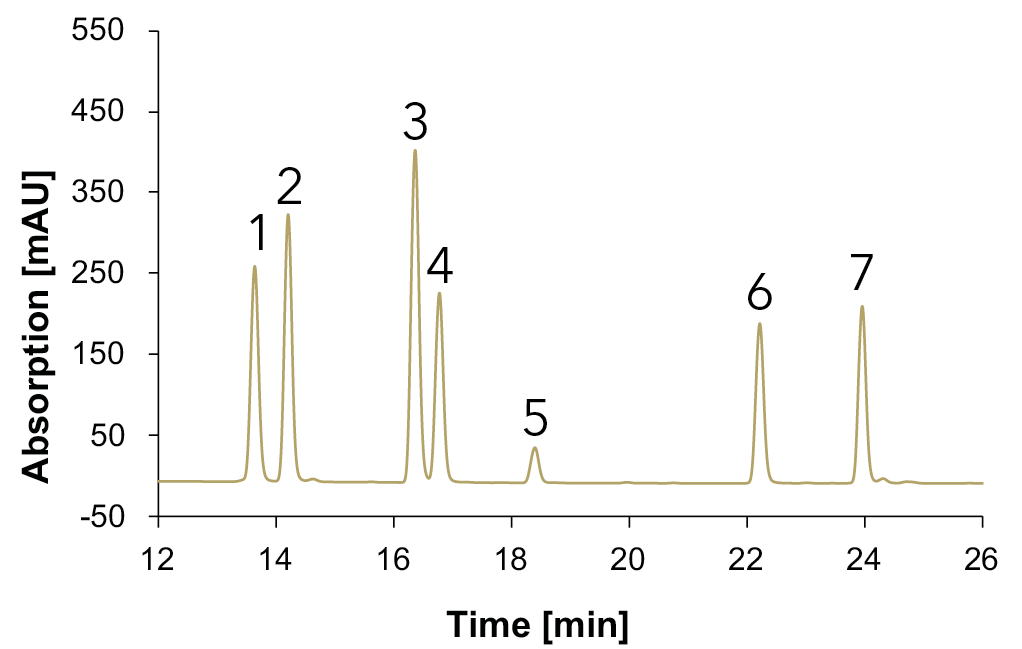
Fig. 14 Chromatograms with standards measured with Eurospher II C18, H2O:MeOH, DAD, 1 ml/min, 1: vanillic acid, 2: syringic acid, 3: p-coumaric acid, 4: ferulic acid, 5: benzoic acid, 6: quercetin, 7: kaempferol; blue: 230 nm, green: 254 nm, gold: 275 nm.
A clear separation of seven phenols/flavonoids is observed, with each substance exhibiting absorption at three distinct wavelengths. This multi-wavelength approach increases substance identification, as different compounds show optimal absorption at specific wavelengths (e.g., benzoic acid (5) at 230 nm, vanillic acid (1) at 254 nm and syringic acid (2) at 275 nm. Additionally, solvent self-absorption at 230 nm underscores the necessity of utilizing multiple wavelengths (254 nm and 275 nm) for accurate identification. An overlay between the chromatograms of the sample and the standard mixture allowed the identification of the compounds in the different samples. The chromatogram for the natural wine is shown in Fig. 15, for the wine 1 in Fig. 16, for the wine 2 in Fig. 17 and for the grape juice in Fig. 18.
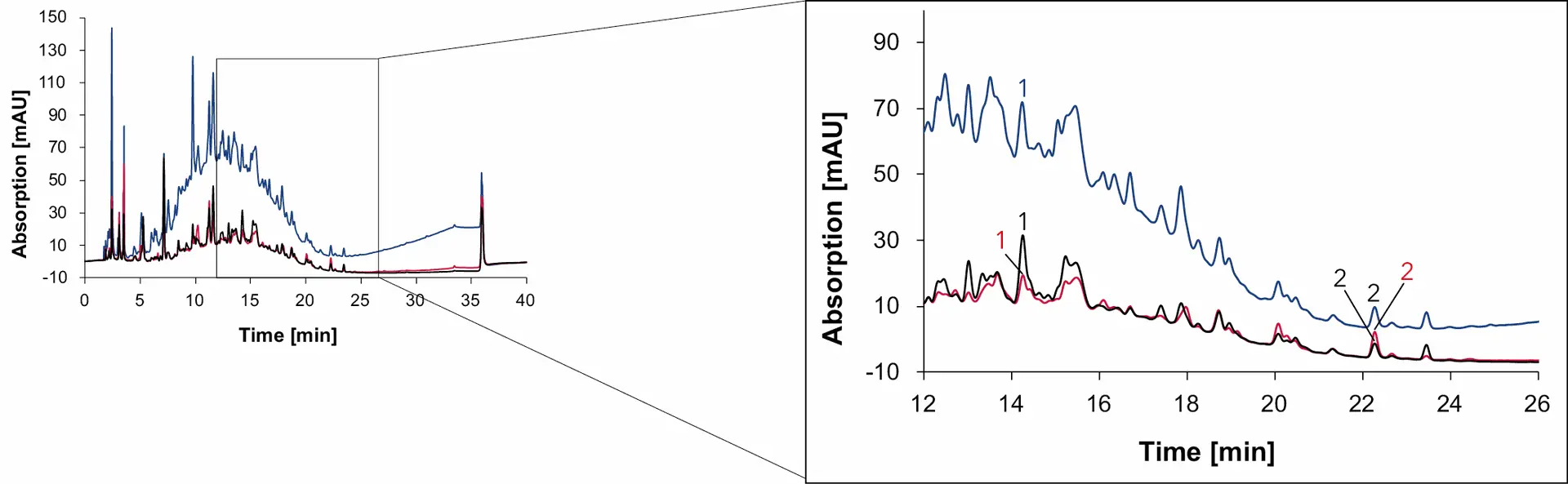
Fig. 15 Chromatogram of natural wine measured with Eurospher II C18, H2O:MeOH, DAD: 230 nm (blue), 254 nm (red) and 275 nm (black), 1 ml/min, 1: syringic acid, 2: quercetin.
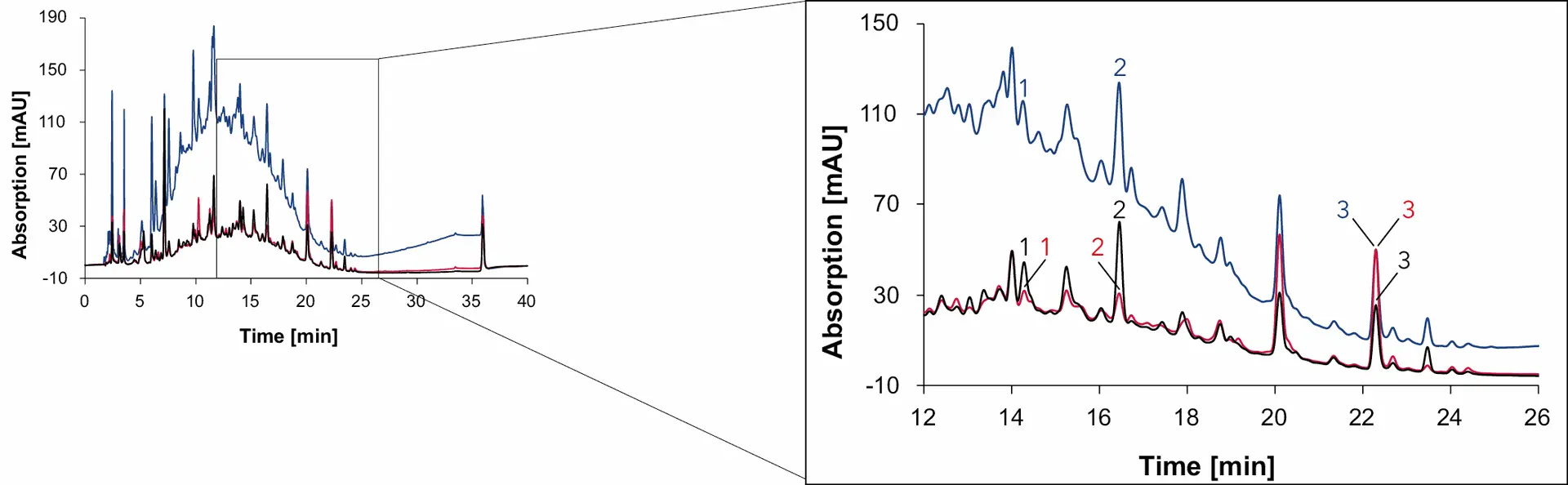
Fig. 16 Chromatogram of wine 1 measured with Eurospher II C18, H2O:MeOH, DAD: 230 nm (blue), 254 nm (red) and 275 nm (black), 1 ml/min, 1: syringic acid, 2: p-coumaric acid, 3: quercetin.
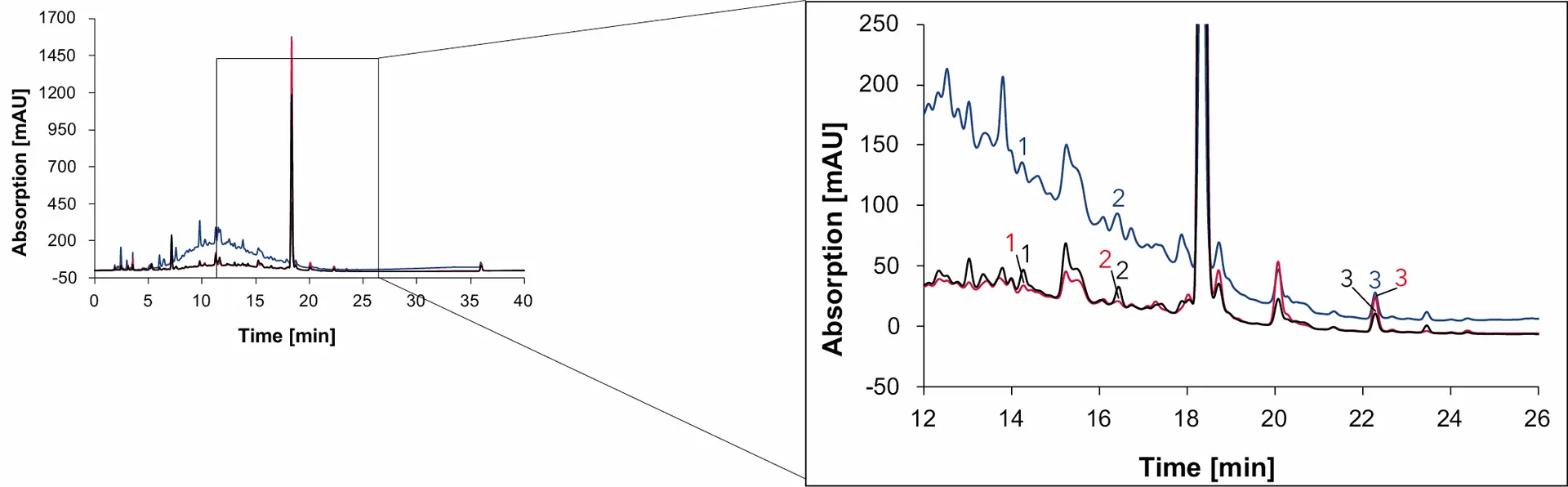
Fig. 17 Chromatogram of wine 2 measured with Eurospher II C18, H2O:MeOH, DAD: 230 nm (blue), 254 nm (red) and 275 nm (black), 1 ml/min, 1: syringic acid, 2: p-coumaric acid, 3: quercetin.
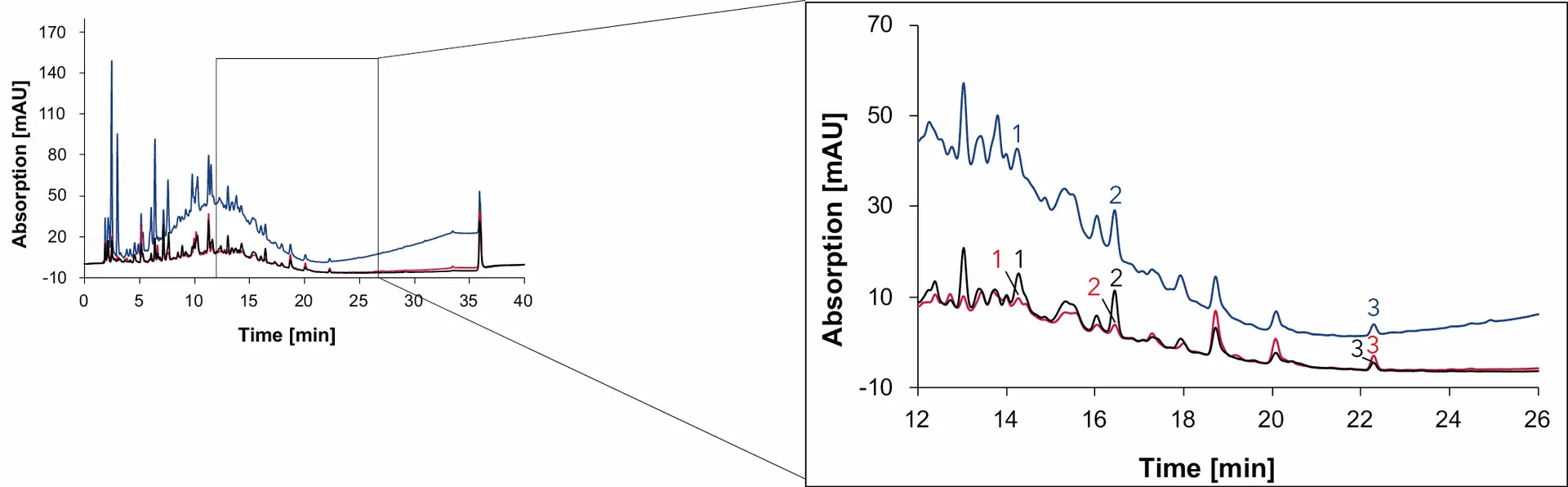
Fig. 18 Chromatogram of grape juice measured with Eurospher II C18, H2O:MeOH, DAD: 230 nm (blue), 254 nm (red) and 275 nm (black), 1 ml/min, 1: syringic acid, 2: p-coumaric acid, 3: quercetin.
Syringic acid and quercetin are contained in the coloring agent of red grapes, that is why both phenolic compounds are found in all four samples [6,7]. In addition, p-coumaric acid was detected in wine 1, wine 2 and grape juice but not in the natural wine. The organic compound, present in grapes and wine, serves as the precursor for 4-ethylphenol, a substance produced by the yeast Brettanomyces in wine [8]. While sulfur is effective in eliminating Brettanomyces, this yeast can easily thrive in natural wines with minimal intervention [9]. Therefore, the absence of p-coumaric acid in natural wine can be explained by the conversion of p-coumaric acid to 4-ethyphenol, resulting in the absence of p-coumaric acid in the natural wine.
As with sugars, organic acids and alcohols, the signal heights of the phenolic compounds in the samples were compared and visualized to give a better understanding of the relative amount of each substance in the different samples (Fig. 19). While the amount of syringic acid does not change significantly between the samples, the amount of p-coumaric acid and quercetin are highly different. The peak signal of quercetin in natural wine is less than 10 mAU, while the signal in Wine 1 is over 50 mAU.
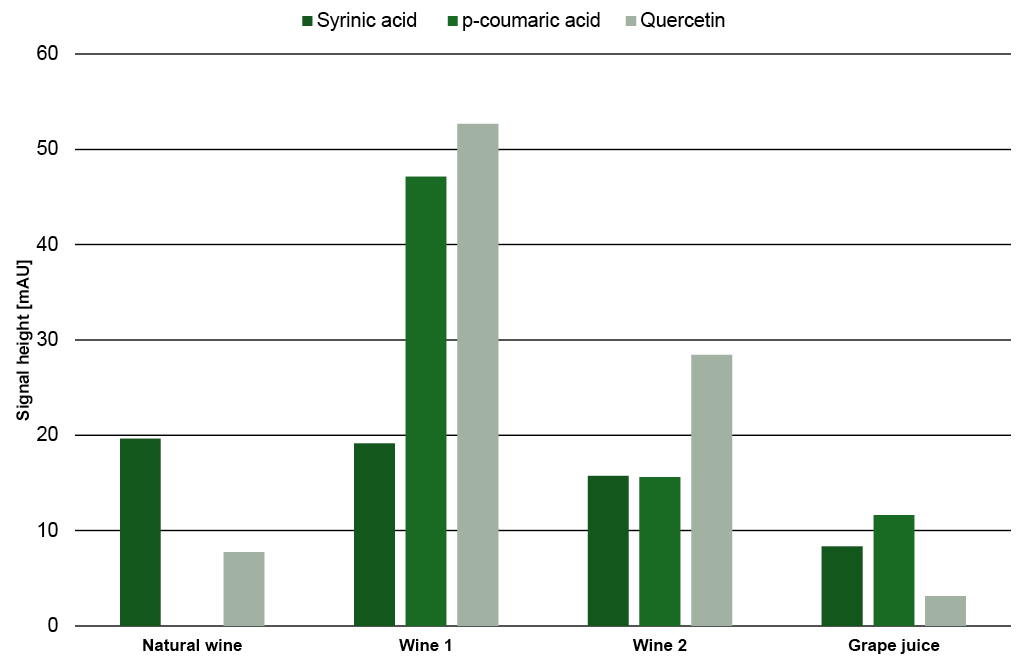
Fig. 19 Signal heights of syringic acid, p-coumaric acid and quercetin in the four different samples.
Conclusion
This application shows two simple and rapid methods for the determination of sugars, alcohols, organic acids and phenolic compounds in wine and grape juice by liquid chromatography. Wine is a complex mixture of hundreds of compounds, each present in a variety of concentrations. HPLC is one possible method for this analysis. An extra sample preparation was not necessary but could lead to a better result. To enable the analysis of various substances the HPLC analysis were performed using a wide range of column materials and detectors. For the determination of the most important organic acids, sugars and alcohols in wine and grape juice, a method using Eurokat columns, RID and DAD was applied. Furthermore, a reversed-phase LC method was developed and optimized for the determination of phenolic compounds.
The analyses were performed on a common C18 reversed-phase column with gradient elution and DAD.
The detection of high levels of glucose and fructose in grape juice and low levels in red wine was as expected. Natural wine was the only sample that contained no sugar. Ethanol was also found in all beverage samples, in high concentrations in alcoholic beverages and in small amounts in non-alcoholic beverages. The detection of organic acids did not differ between the wine samples, but there were clear differences compared to grape juice. For example, acetic acid and succinic acid were not found in grape juice, whereas malic acid was found in grape juice but not in wine samples. The phenolic compounds were similar among the samples, with the exception of p-coumaric acid which, as expected, was the only substance not detectable in natural wine. In summary, a dedicated system with two detectors enables the detection of the most commonly substances in beverage samples such as wine and juice.
Material and Methods
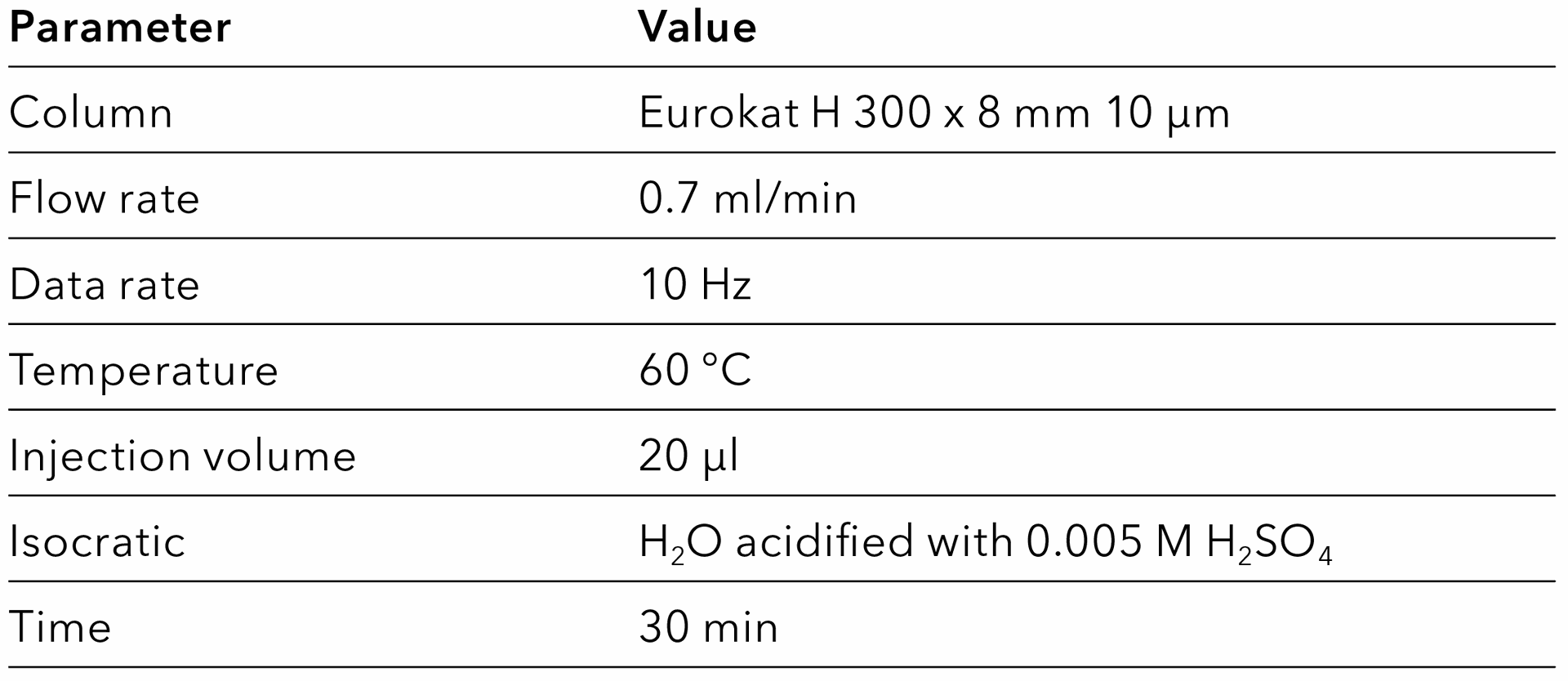
Tab. 2 Method for the Eurokat H column.
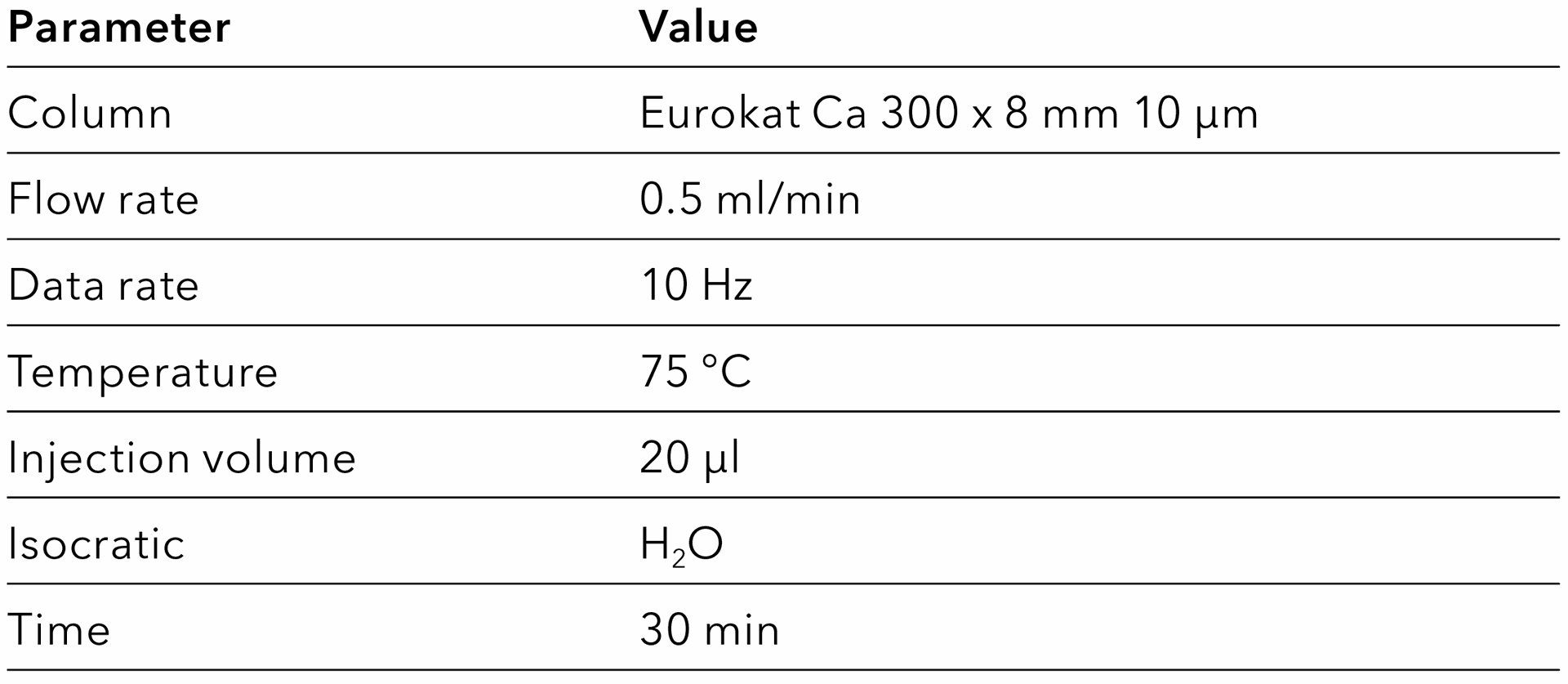
Tab. 3 Method for the Eurokat Ca column.
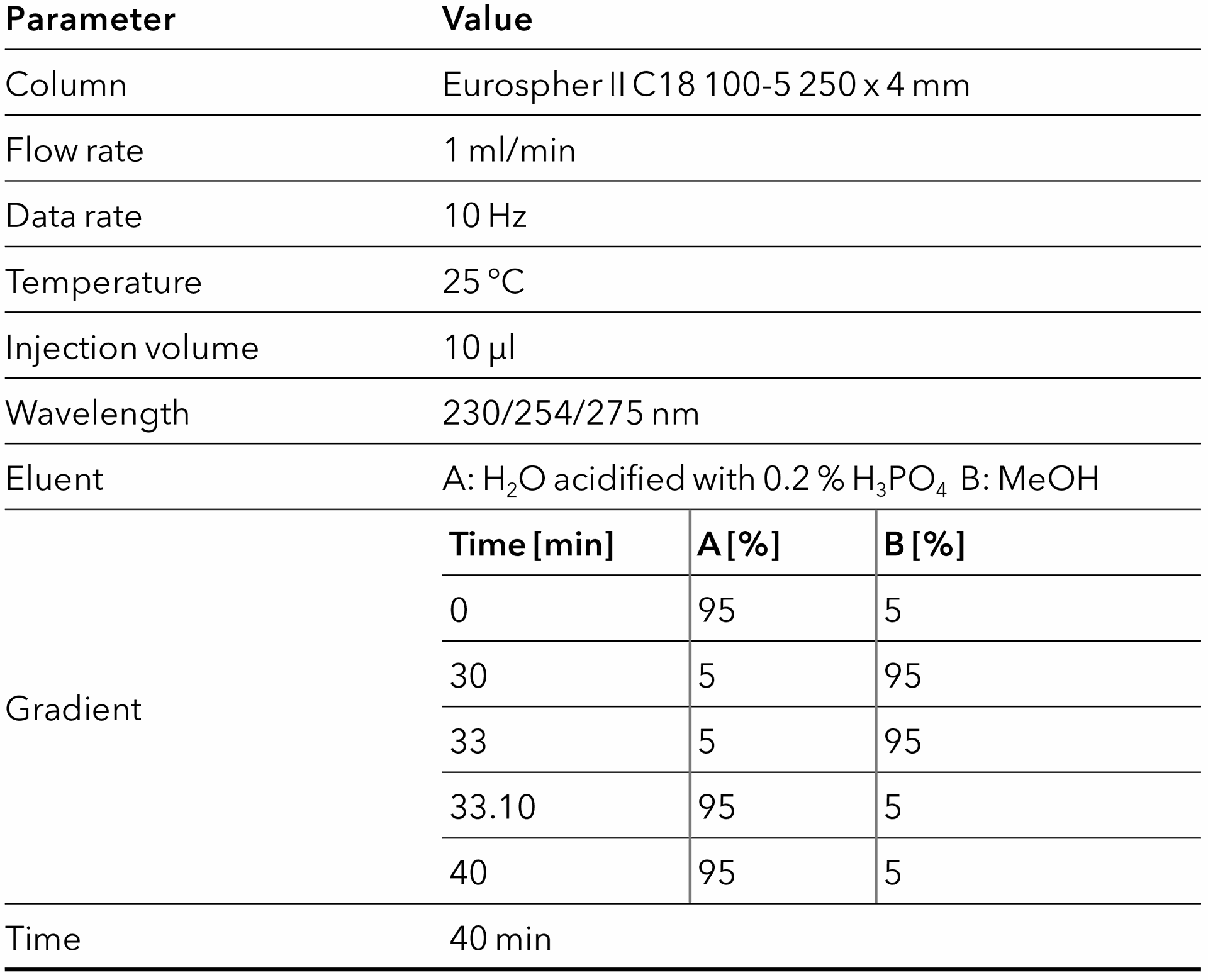
Tab. 4 Method for Eurospher II C18 column.
Tab. 5 System configuration.

Fig. 20 System setup; from top to bottom: eluent tray with bottles, pump, diode array detector (DAD), refractive index detector (RID), autosampler, right: oven.
References
[1] Kelebek, H., Selli, S., Canbas, A. & Cabaroglu, T. (2009). HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchemical Journal, 91(2).
[2] Webendorfer, U., Haim, K. (2013). Weinanalyse. Pädagogische Hochschule OÖ (Fachdidaktikzentrum Naturwissenschaften).
https://www.yumpu.com/de/document/
view/10541925/weinanalysen
[3] Kerem, Z., Bravdo, B., Shoseyov, O. & Tugendhaft, Y. (2004). Rapid liquid chromatography–ultraviolet determination of organic acids and phenolic compounds in red wine and must. Journal of Chromatography A, 1052(1–2), 211–215.
[4] LC Technical Note LT192: Analysis of malic acid by HPLC. GL Sciences.
https://www.glsciences.com/viewfile/?p=LT192
[5] Vodnar, D. & Socaciu, C. (2008). Comparative analysis of lactic acid produced by apple substrate fermentation. Using HPLC and Tectronic Senzytec biosensor. Bulletin of The University of Agricultural Sciences And Veterinary Medicine Cluj-Napoca, 65(2), 444–449.
[6] Georgiev, V., Ananga, A. & Tsolova, V. (2014). Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients, 6(1), 391–415.
[7] Carneiro, C. N., Gomez, F. J., Spisso, A., Silva, M. F., Azcarate, S. M. & De S Dias, F. (2020). Geographical characterization of South America wines based on their phenolic and melatonin composition: An exploratory analysis. Microchemical Journal, 158, 105240.
[8] Salameh, D., Brandam, C., Medawar, W., Lteif, R. & Strehaiano, P. (2008). Highlight on the problems generated by p-coumaric acid analysis in wine fermentations. Food Chemistry, 107(4), 1661–1667.
[9] Branco, P., Coutinho, R., Malfeito-Ferreira, M., Prista, C. & Albergaria, H. (2021). Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide. Microorganisms, 9(12), 2528.
Application details
Method | HPLC |
Mode | Ion exclusion, Ligand exchange, RP |
Substances | Sugars, alcohols, organic acids and phenolic compounds |
Key words | HPLC, beverage analysis, qualification, RID, DAD, wine, juice, organic compounds |
CAS number | 57-50-1, 50-99-7, 57-48-7, 56-81-5, 64-17-5, 50-70-4, 87-69-4, 6915-15-7, 138-59-0, 79-33-4, 64-19-7, 77-92-9, 110-15-6, 530-57-4, 121-34-6, 501-98-4, 1135-24-6, 65-85-0, 117-39-5, 520-18-3 |
Version | VFD0194 | version 1 09/2024 | ©KNAUER Wissenschaftliche Geräte GmbH |
Documents
Here you have the possibility to download the application.



