The influence of metal ions in HPLC has been known for some time; we are talking more about a problem that exists but has been less of a focus for many users to date. In recent years, however, this topic has experienced something of a renaissance due to the increasing importance of biomolecules.
Which metal ions are involved?
Where do they come from and what do they do?
Can they be avoided? And can they be removed?
But before we turn our attention to this topic, let's first take a brief semantic digression:
Confusion of terms
Terms in this context are not used consistently by all parties involved. This creates a certain amount of confusion. Below is a brief classification, although this does not claim to be exhaustive, nor can I expect everyone to be 100% in agreement with it.
- Bioinert; low interaction with hardware components, low risk of carryover; oldest term, used as early as 1933: Aluminum surface as an alternative to iron. In the HPLC environment, the materials used here are titanium, ceramic, PEEK
- Biocompatible; no risk of corrosion despite chloride ions in the eluent; often synonymous or equated with Bio-LC and stainless steel-free; materials used: titanium and special alloys, e.g. MP35N
- Metal-free; materials used: PEEK, sapphire
Other attributes to be found are: “Inert”, ‘truly bioinert’ (PEEK), finally ‘fully biocampatible’.
More accurate descriptions would be: “Low Adsorption Materials” or “Corrosion Resistant Materials” - but that is a personal opinion.
1. Where do they come from?
- In older stationary phases, heavy metal ions are present as contaminants in the silica gel matrix. The first-generation silica gel used is of natural origin and is therefore subject to natural fluctuations. This means that heavy metal ions, but also K, Na, Ca, Al etc. are present in the silica gel framework, see Figure 1
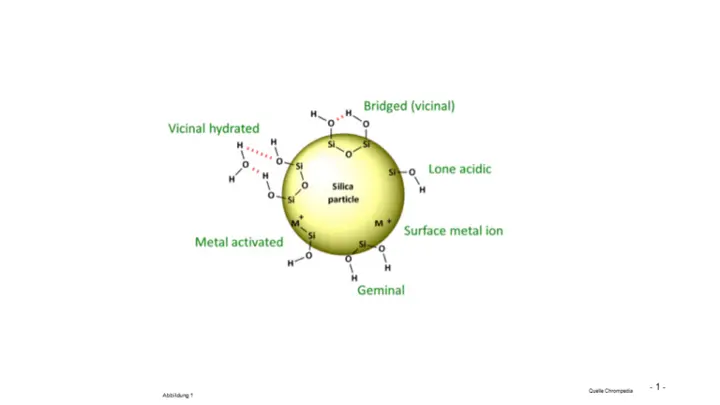
Figure 1 - Metal ions in silica gel for HPLC
- All solvents used in HPLC wash out metal ions from metal-assembled parts in the system, see Figure 2-4
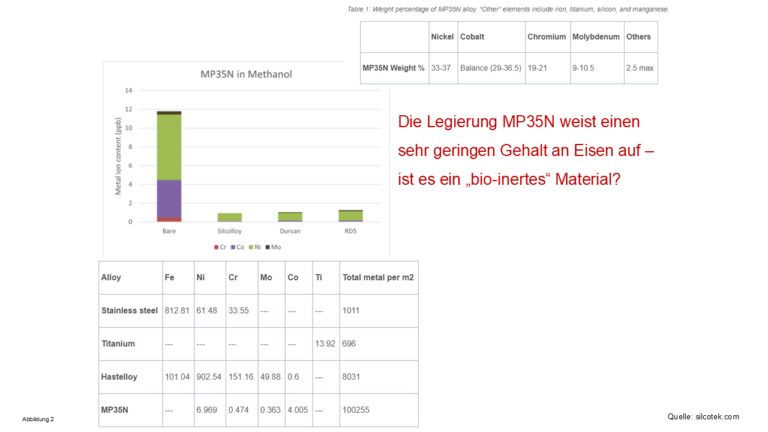
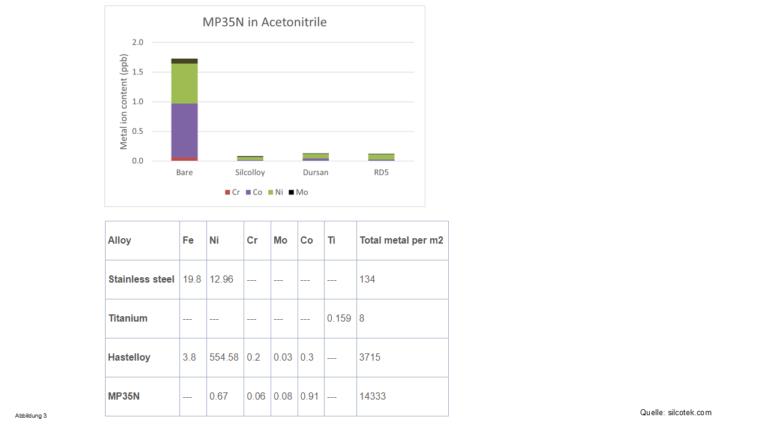
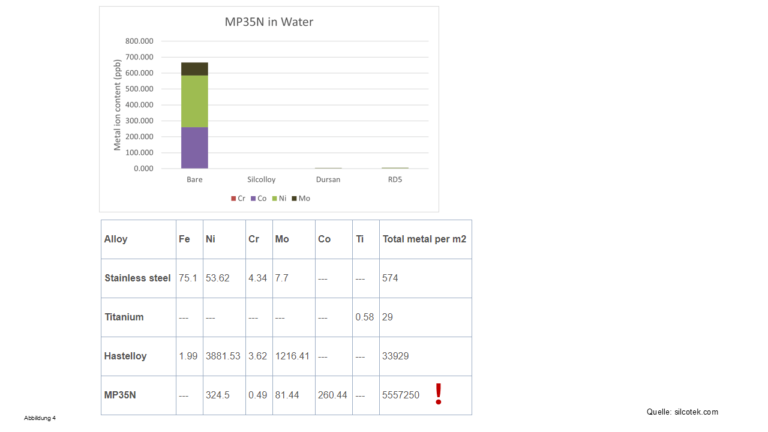
Figure 2-4 - Metal ions washed out by HPLC solvents
- Acetonitrile has the lowest “leaching power”
- Even the seemingly harmless water “properly” washes out metal ions out
- The alloy MP35N does not contain any iron ions, but the other metal ions
metal ions contained in it are washed out by all solvents washed out by all solvents - especially water, see the amount of metal ions washed out in Figure 4!
amount of metal ions washed out! When using such a material in an HPLC
material in an HPLC system be called “Bio-LC”? (Bio)molecules can also complex with
with metal ions other than iron!
- Metal ions enter the device together with the sample and reach the column, where they are adsorbed in/on the stationary phase. Just think of contrast agents (barium, gadolinium) and cytostatics (platinum ions)
2. What do they do? Here are some examples:
- On the one hand, metal ions on the silica gel surface can form metal complexes, see Figures 1 and 5. On the other hand, heavy metal ions inside the silica gel matrix exert an -I effect on the silanol groups (they withdraw electrons from them) and thus promote the formation of dissociated (ionic) and therefore “aggressive” silanols. These can enter into ion exchange interactions with basic molecules, resulting in chemical tailing. We have seen above that the older silica gels contain a considerable amount of metal ions as contaminants. This is the reason why chemical tailing always occurs with older columns despite the use of an acidic eluent - it is almost impossible to avoid “aggressive” silanols with such materials
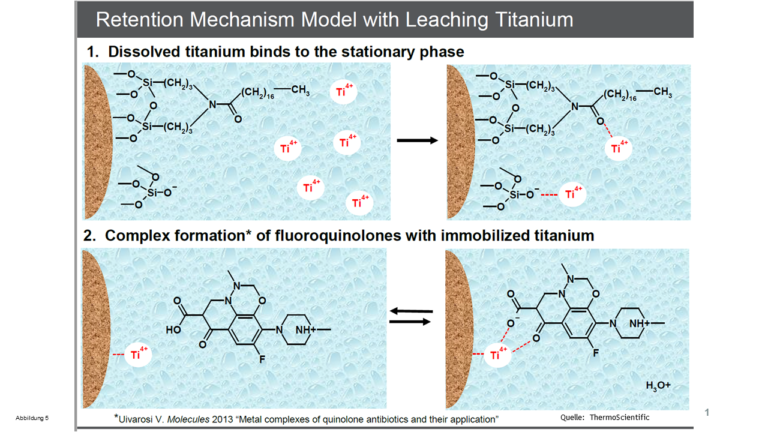
Figure 5 - Influence of metal ions in the silica gel matrix
- Biomolecules, but also other molecules (Lewis bases, acids) are partially adsorbed on metal surfaces, result: no reproducible peak areas, strong tailing, in worst case total adsorption (memory effect, carryover)
- Fe2+ ions can form complexes (carboxylates, N-containing substances with a free electron pair on nitrogen), they also have a catalytic effect in a number of reactions
- Quinones can be reduced to catecholamines by Fe2+ ions
- There is a general risk of a biofilm forming in an HPLC system, especially if buffers are used frequently. Bacteria produce sugar compounds, which in turn form a polymer matrix. This “soaks up” (also) heavy metal and alkali ions; biomineralization creates a hard plaque that is difficult to remove. Result: Tailing and/or “hump”
3. Can they be avoided?
Hardly: As long as solvents come into contact with metallic surfaces - whatever they may be - metal ions will be washed out in certain concentrations. The extent to which this process is harmful depends on the molecules. In the meantime, there are already PEEK-coated columns (pressure stability?) or coated columns, e.g. amorphous glass. The molecules therefore do not “see” any metal surface in the column - but only in the column. And note in this context: the larger the (specific) surface area, the more metal ions are washed out of it, so the frit, for example, deserves special attention. Efforts have been underway for some time, but to date there are (still) no commercially available, completely metal-free HPLC systems.
4. Can they be (completely) removed?
Here, too, the answer is: probably not. Of course, rinsing procedures with EDTA and phosphoric/oxalic acid etc. are known and have been described. Apart from practical problems, such a step is helpful, but it can never lead to a complete and above all sustainable removal of metal ions. Is it not at least possible to “hide” metal ions in the apparatus? Yes, that is also possible, but this is hardly a satisfactory solution in the long term: Passivation with 6 N HNO3 definitely helps for a while - but only for a while: For a while. The procedure is somewhat time-consuming and would have to be repeated 2-3 times a year. So, passivate: Perhaps, alternatively, possibly, possibly and as a “last resort” already worth considering. The removal of a possible biofilm is a matter in itself, which we will deal with in a future HPLC tip.
We've been bashing the “poor” metal ions all this time. Is there nothing positive to say about them? Yes, there is: certain chiral columns can be deliberately coated with Cu2+ or Cd2+ ions. These cations form large complexes with the chiral selectors on the surface of the material and the enantiomers to be separated. Acetonitrile molecules cause chelate stabilization, resulting in excellent enantioselectivity (“ligand exchange chromatography”). But I have to admit that this is something special...
® Dr. Stavros Kromidas
Further tips from this year can be found under: https://www.kromidas.de/hplc-tipps-des-jahres/

